Center for Advanced Design and Manufacturing of Integrated Microfluidics (CADMIM)
October 16, 2020

Singling out a diseased or mutated cell and identifying which treatment option is most effective can be cumbersome, expensive, and time-consuming. Currently, the process involves having a centralized lab with large robotic systems to test various solutions.
A team of researchers have discovered a way to get test results on individual cells in hours, not days, and at a fraction of the cost. Fast and affordable drug testing increases the potential for personalized treatments. Their work is a part of the Center for Advanced Design and Manufacturing of Integrated Microfluidics (CADMIM), an Industry-University Cooperative Research Center (IUCRC) funded by the National Science Foundation.
The research team is leveraging microfluidics—technologies for manipulating tiny amounts of fluids—to enclose a single cell into a droplet of fluid for testing. Droplet microfluidics occur on computer chip-like devices, explained Abraham Lee, lead investigator and a biomedical engineer at University of California, Irvine. Electric currents zip across a chip through channels. With microfluidics, fluids flow through microchannels that are 1-100 microns wide (a human red blood cell is about 5 microns, while a sheet of paper is about 100 microns thick).
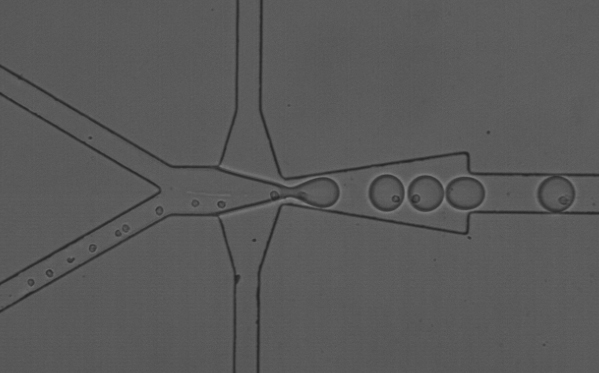
The team used microfluidics to put a cell into a droplet of reagent (a chemical or drug), along with a bead that acts like a mini barcode for the combination. “We call it the 1-1-1 technology,” said Lee. “It’s like its own little test tube.” The new technology allows researchers to quickly screen libraries of chemical compounds, leading to more rapid drug screenings. The process would ultimately lower the lab footprint, automate much of the experiment, and lower drug development costs.
Increasing the 1-1-1 Efficiency
In the past, cancer research meant taking a sample of a tumor, grinding it up, and performing tests on the cell slurry. The research results reflected the average of all cells in the sample, not just the cancer cells.
Over the past decade, researchers discovered how to hone in on a single—possibly problematic—cell, allowing them to test which treatments worked best on that cell. “Droplet microfluidics allows you to actually separate out single cells into these droplets without killing the cell,” said Lee.
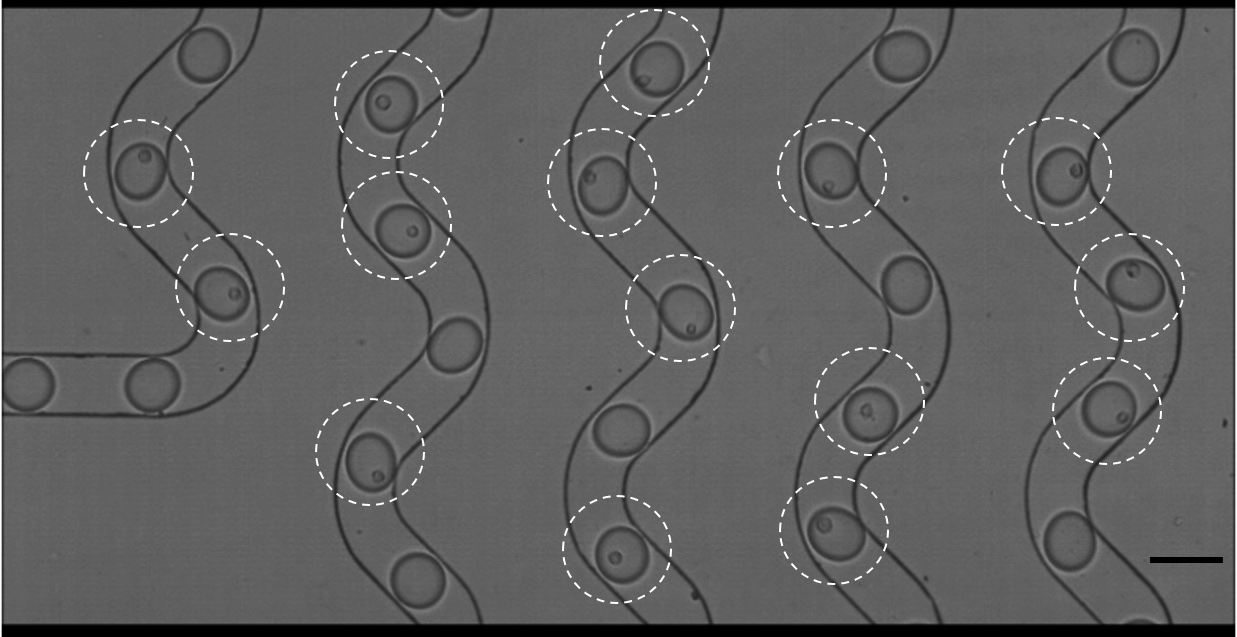
1-1-1 technology enables researchers to observe how a cell will react in different conditions—useful when tailoring treatments for a patient. But the same micro channels that allow for precise cell encapsulation are the very thing that hampered efficiency and cost at larger scales.
Lee explained that backups occurred as cells traveled through microchannels. Picture people standing in line to board a bus—if everyone tries to get on at the same time, sometimes multiple people board at once, and sometimes none get on. The cells behaved the same way when trying to get into a droplet, leaving it blank.
“You can end up burning through expensive reagents or it takes a very long time to get all the cells into droplets,” said Lee, adding that the pile up meant that the process was only about 10% efficient.
Lee and his team found that if they created certain circumstances—positioning the cell just right and ensuring a perfect current flow—the cell would be sucked into the channels one at a time. Their method raised encapsulation to 50% efficiency.
Single Cells Research and Cell Communication
Understanding how cells communicate with each other has implications for certain diseases, like cancer. For example, with cancer immunotherapy treatments, doctors try to boost the immune system of a patient to target specific infections or diseased cells. The technology can evolve from single cell research to putting multiple cells into close proximity, or even in the same droplet.
“This process is still very difficult to study because you need a healthy cell that’s in the act of doing some sort of defense process,” said Lee. Using droplet bead technologies, scientists can put immunity T-cells with the problematic cell and study the interactions and responses.
Having these breakthroughs in microfluidics is critical in corporate laboratories. Yue Yun, the Enabling Transformational Technologies Lead at Corteva Agriscience and CADMIM Industrial Advisory Board member, said that the collaboration with university researchers “generated a promising proof-of-concept device.” Yun added that the working prototype for a potential microfluidic instrument could help corporate laboratories remain competitive.
